Usp 797 changes with engineering controls removal of references to hazardous drugs defined temperature relative humidity requirements 20 c 68 f or cooler 60 rh no in room humidifiers or de humidifiers defined interval for environmental monitoring monthly 5.
Usp 797 clean room temperature and humidity requirements.
While usp 795 and usp 797 focus on the quality of patient outcome by producing compounds.
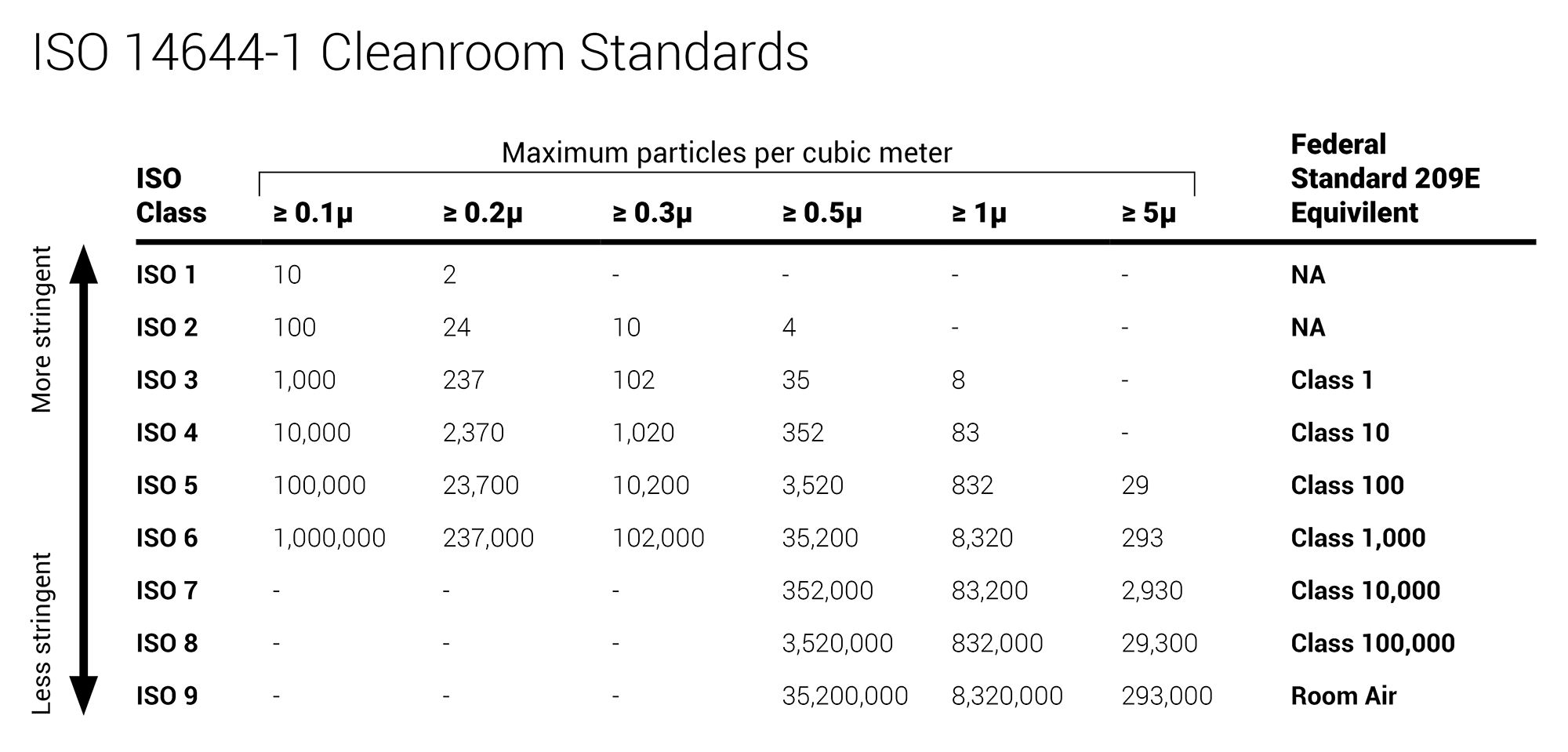
Secondary engineering control requirements by usp chapter 797 risk level low risk l ante area an iso class 8 see table 21 1 or cleaner area where personnel hand hygiene and.
In june 2019 the united states pharmacopeia usp released several new and revised pharmacy compounding standards.
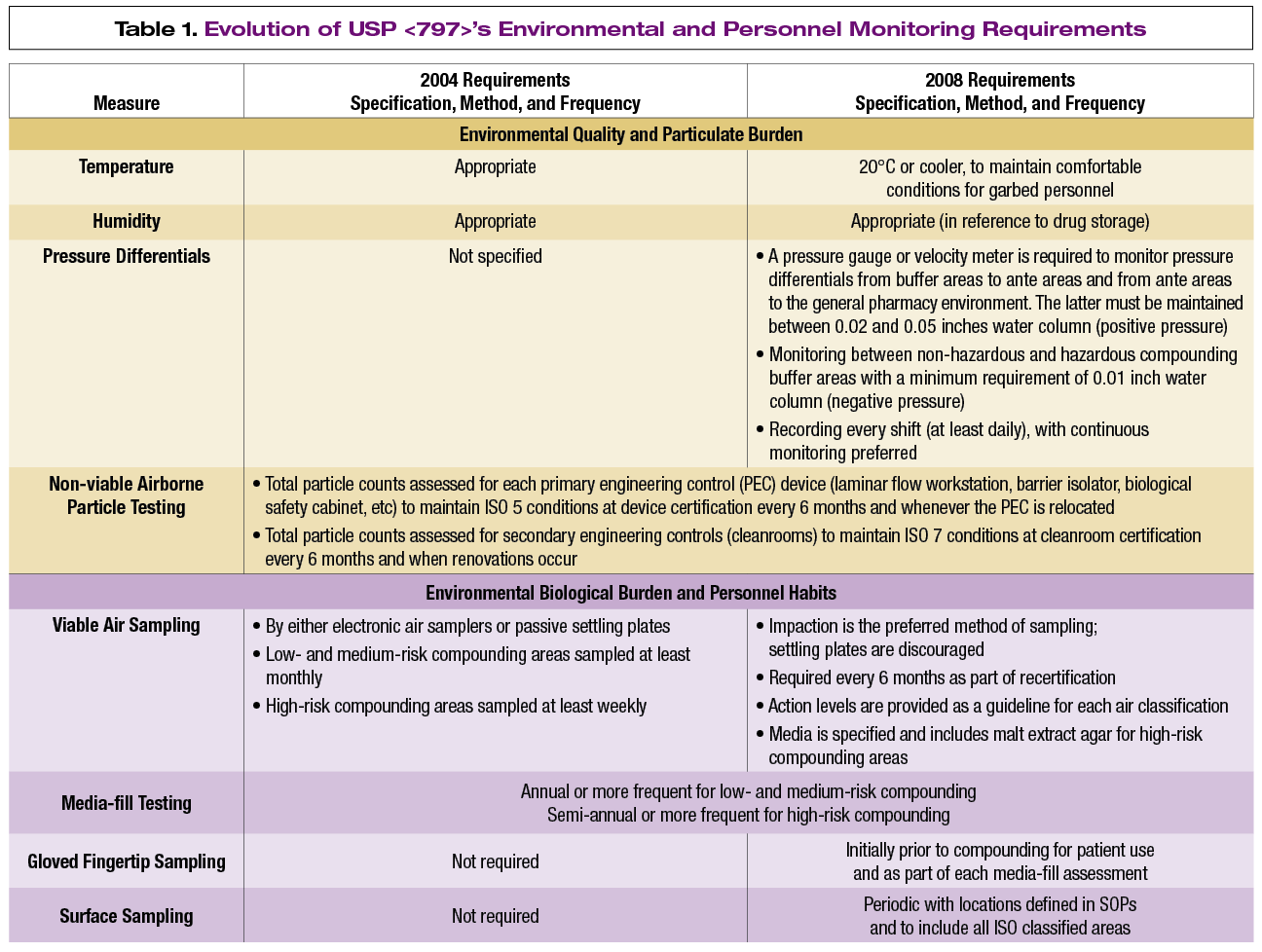
Temperature and humidity gauges mounted in the room see appendix 1.
The design of stability studies during pharmaceutical product development and registration takes into account expected supply chain storage and distribution conditions in anticipated markets.
Standards for compounding sterile preparations usp 797 helps to ensure patients receive quality preparations that are free from contaminants and are consistent in intended identity strength and potency.
Minimum standard for pharmacies in hospitals.
33 other professional organizations also provide guidance on specific aspects of compounding.
Standards for prescribing preparation.
Temperature and humidity requirements for the c sec room are lower than in the past.
Maintaining usp 800 s required acph engineering and iso clean air standards.
Revision as yet unproposed in pf of usp s definition for controlled room temperature from 20 25 to 2 30.
These requirements are addressed in usp 797 with revisions that call for a standard of a continuously maintained temperature of 20 degrees c 68 degrees f and 60 percent humidity.
5 as described in usp chapter 797 for exposure of critical sites and must be in table 21 2.
Inside the room and in which other relevant parameters e g temperature humidity and pressure are controlled as necessary it is critical to understand how your pharmacy cleanroom is impacted by the myriad of standards and references for cleanroom de sign and operation including usp chapter 797 require.
Personnel validation three consecutive media fill runs without contamination.
Low to medium risk.
Usp 797 guidelines minimum requirements for validation.
Usp 797 clean room guidelines standards.
Practices28 31 32 and a discussion guide on usp chapter 797 17 and has recognized usp chapter 797 as a relevant practice standard in the ashp guidelines.